UX design for FDA-cleared neuroimaging software
We helped a medical technology company handle the UX improvements of their product and get successful FDA clearance to enter the US market.
Context
Our customer, NordicImagingLab, is a sister-company of NordicNeuroLab — an innovative medical technology company specializing in the development, validation, sales, and distribution of healthcare solutions. NordicImagingLab was founded in 2019 as a spin-out of NordicNeuroLab, and together they offer products to companies worldwide. NordicImagingLab focuses on developing software for image processing of functional magnetic resonance imaging (fMRI) and diffusion tractography MRI, which are both key techniques to support advanced neurosurgical interventions.
The customer’s solution enables automation and accuracy in diagnosing neurological conditions. NordicImagingLab’s primary clientele is hospitals where neurosurgeons and radiologists utilize their technology for pre-surgical planning, tumor evaluation, follow-ups after treatment, and more.
The customer needed a technology partner to handle the improvement of their solution’s user experience end-to-end, from documenting requirements to introducing changes based on user feedback. In addition, they needed assistance with managing the Food and Drug Administration (FDA) clearance as the customer planned to enter the US market. Considering Itransition’s experience with healthcare software development and UX/UI design, the customer decided to partner with us.
Solution
Working with users & elaborating UX requirements
Itransition's UX/UI team started by delving into the details of the customer’s product and target audience to understand all the design requirements, working closely with the customer’s project manager, product owner, and CEO.
Our team created the description of user groups, focusing on radiologists and neurosurgeons as primary target groups. Other users included researchers, radiographers/MR technicians, managers responsible for buying decisions, and IT admins working for top-tier medical institutions in the US. Itransition’s expert created a customer journey map documenting users’ main goals, tasks they would perform with the help of the customer’s solution, and possible work-related problems.
Then, Itransition analyzed the existing design and went on to plan and conduct user sessions with radiologists and neurosurgeons recruited by the customer. We documented user requirements discussed during the sessions in line with FDA requirements and the customer’s Quality Management System (QMS). By holding these exploratory interviews before starting the design works, we ensured that the design would fully align with user needs.
The interview results were documented and transformed into design and usability requirements. User interviews, documentation, and follow-up were conducted following recognized standards for usability engineering, specifically ISO 62366. To ensure consistent solution improvement in line with user needs, the interview results were converted into backlog tasks. The interviews became part of the development process, adding to the transparency of where feature ideas stemmed from and how the tasks connected to those ideas were prioritized. Together with the product owner, our team began describing requirements as user stories, which also added clarity to reasons why users needed certain functionality, while balancing patient safety and security requirements.
Improvements delivery
One example of improvements we made after an exploratory interview was changing the landing page based on user feedback to rectify the fact that users rarely went back to previous sessions while they occupied a lot of space on the main screen.
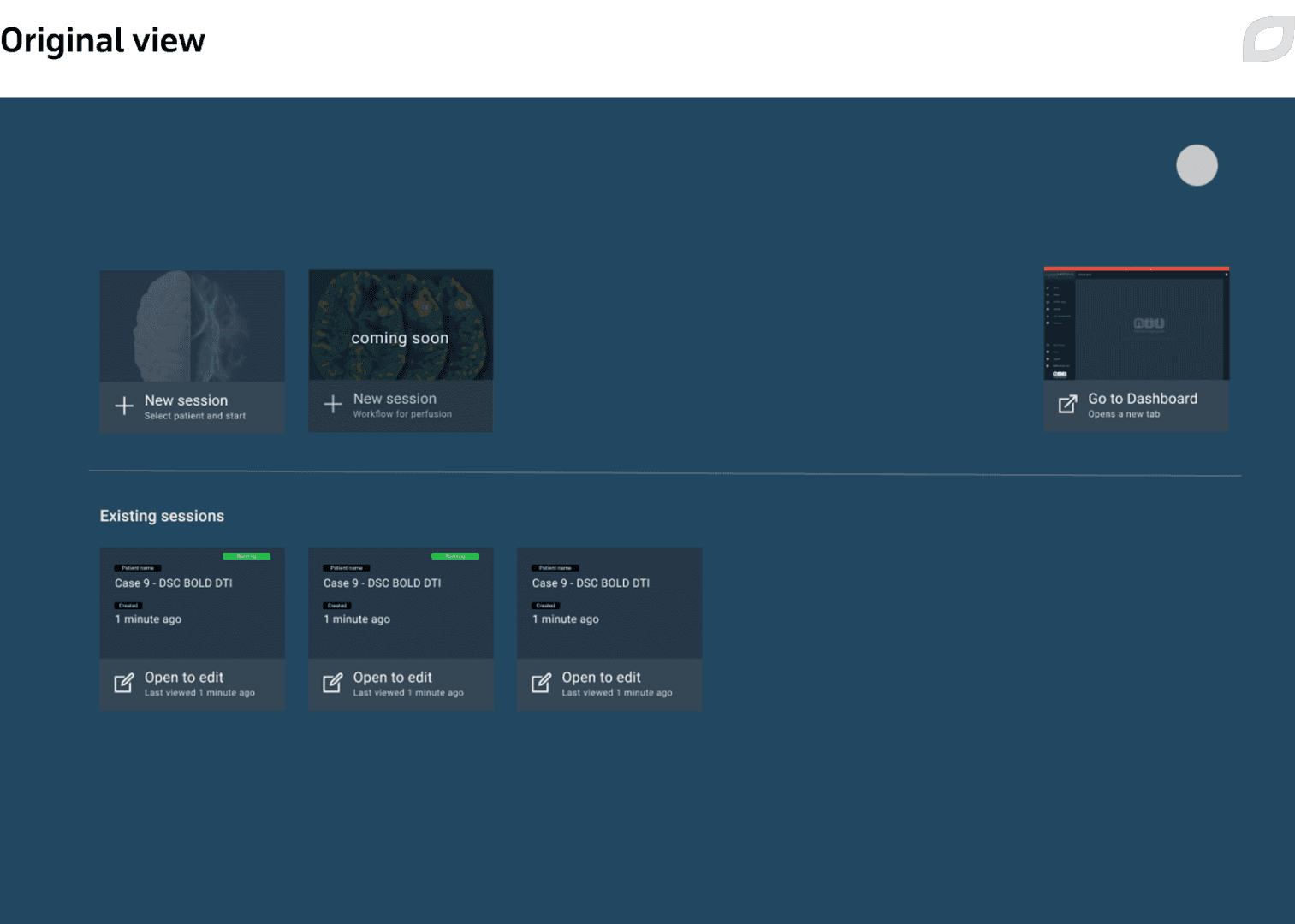
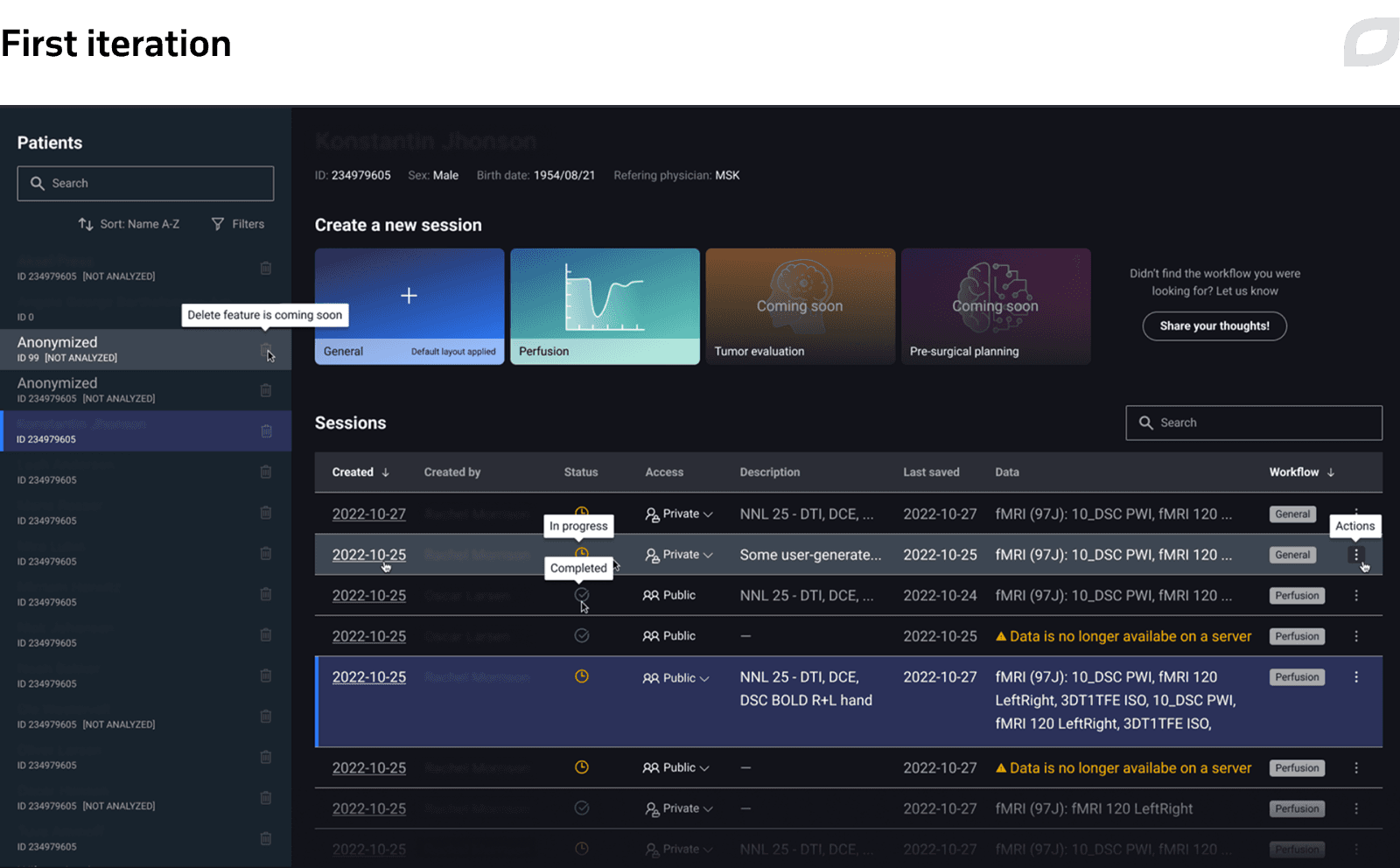
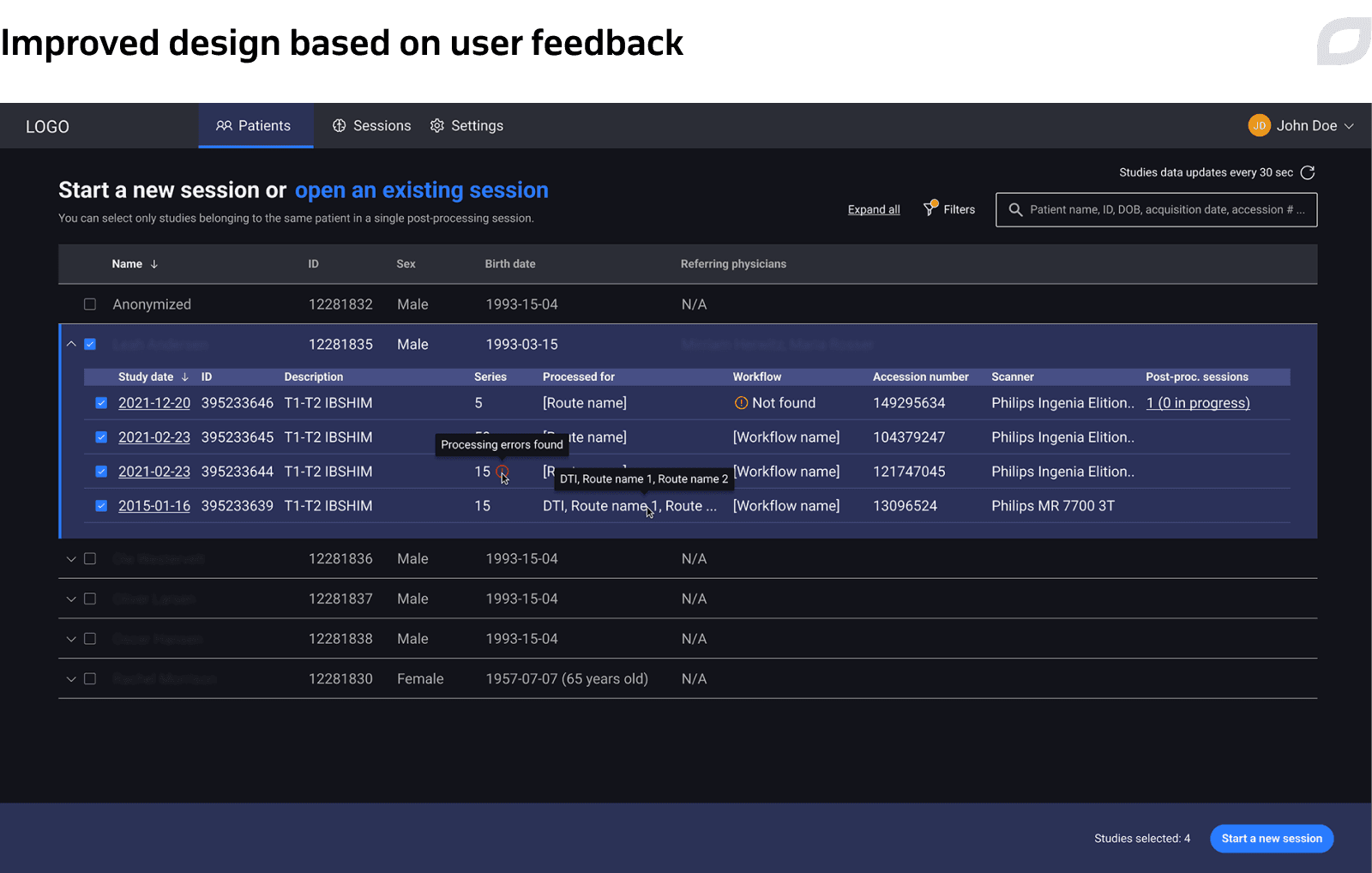
Another example of design improvements included redesign of the Viewer’s layout, the application’s main page. We refreshed the visual style, updated the top menu, and reworked the patient data widget to enable faster, more convenient interactions, which is crucial in the clinical setting.
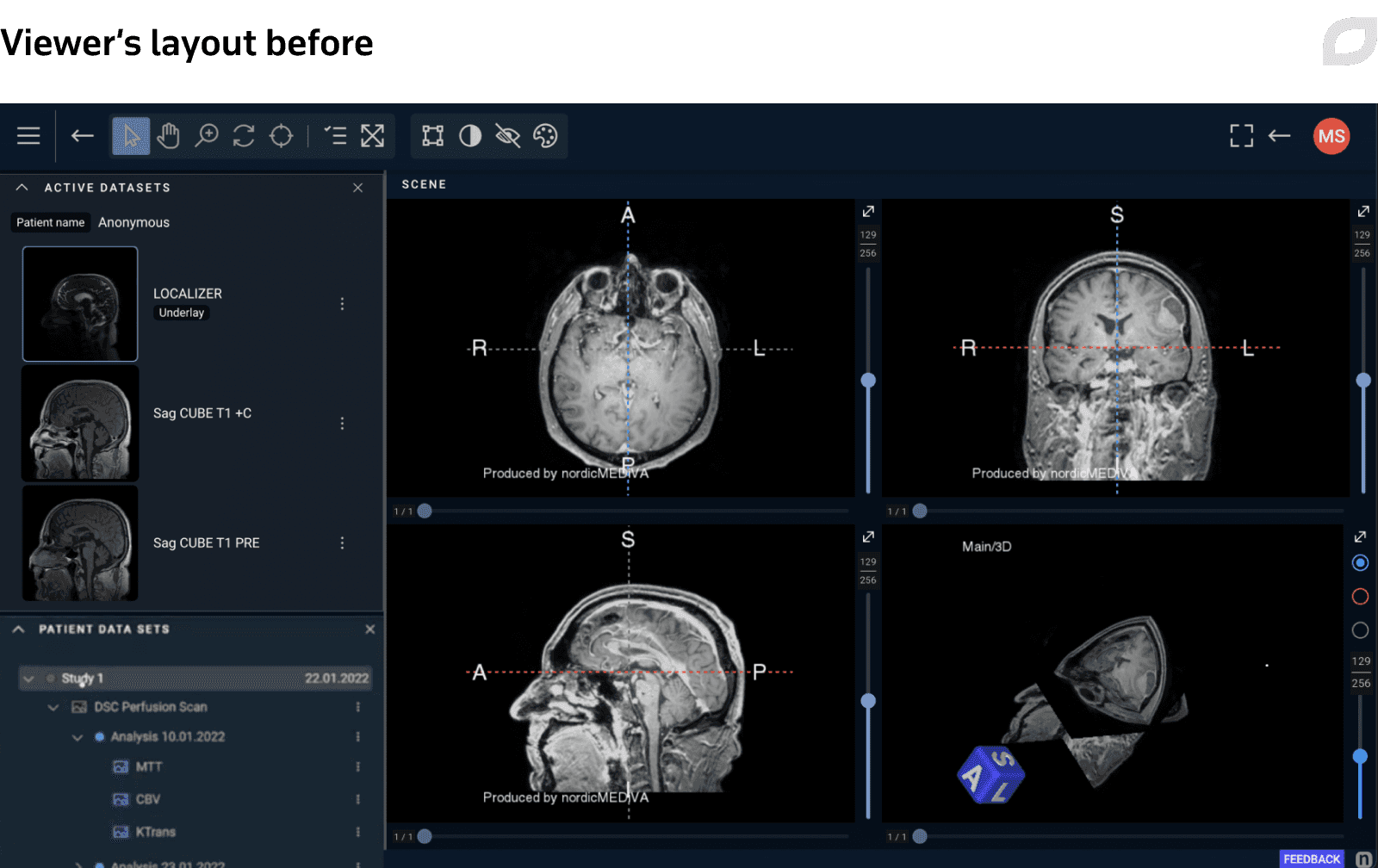
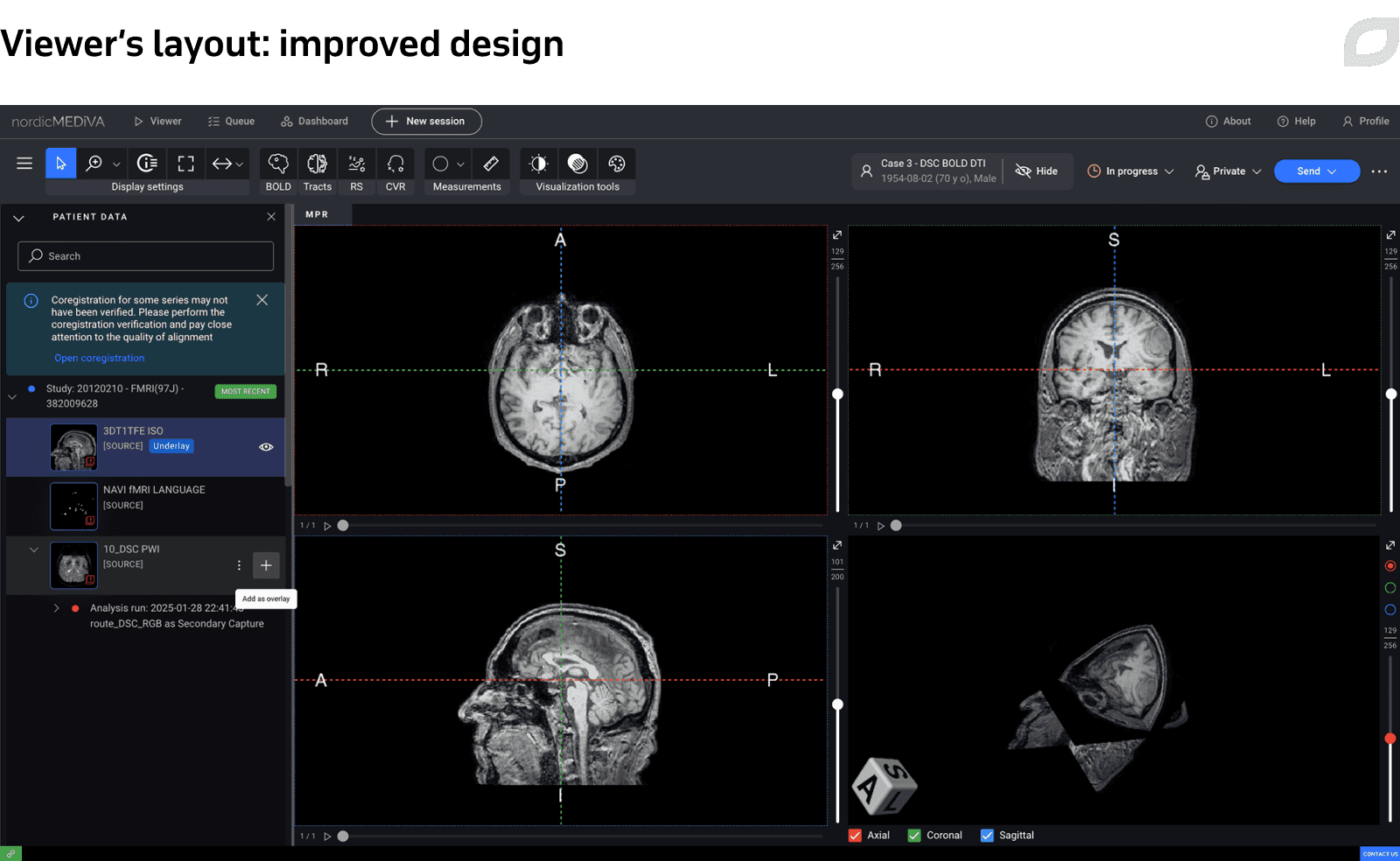
To streamline the design and development, Itransition UX/UI experts discussed possible technical constraints and design feasibility with frontend developers on the customer’s side who advised us how to optimize and accelerate design delivery. We also worked with the product owner to prioritize design improvements.
Together with the customer, our expert also prepared detailed user instructions which, besides simplifying the users’ onboarding, were part of the FDA-required documentation. The instructions covered the information on the software’s installation and settings, working with patient data and Digital Imaging and Communications in Medicine (DICOM) nodes, and more.
Website & event stands design
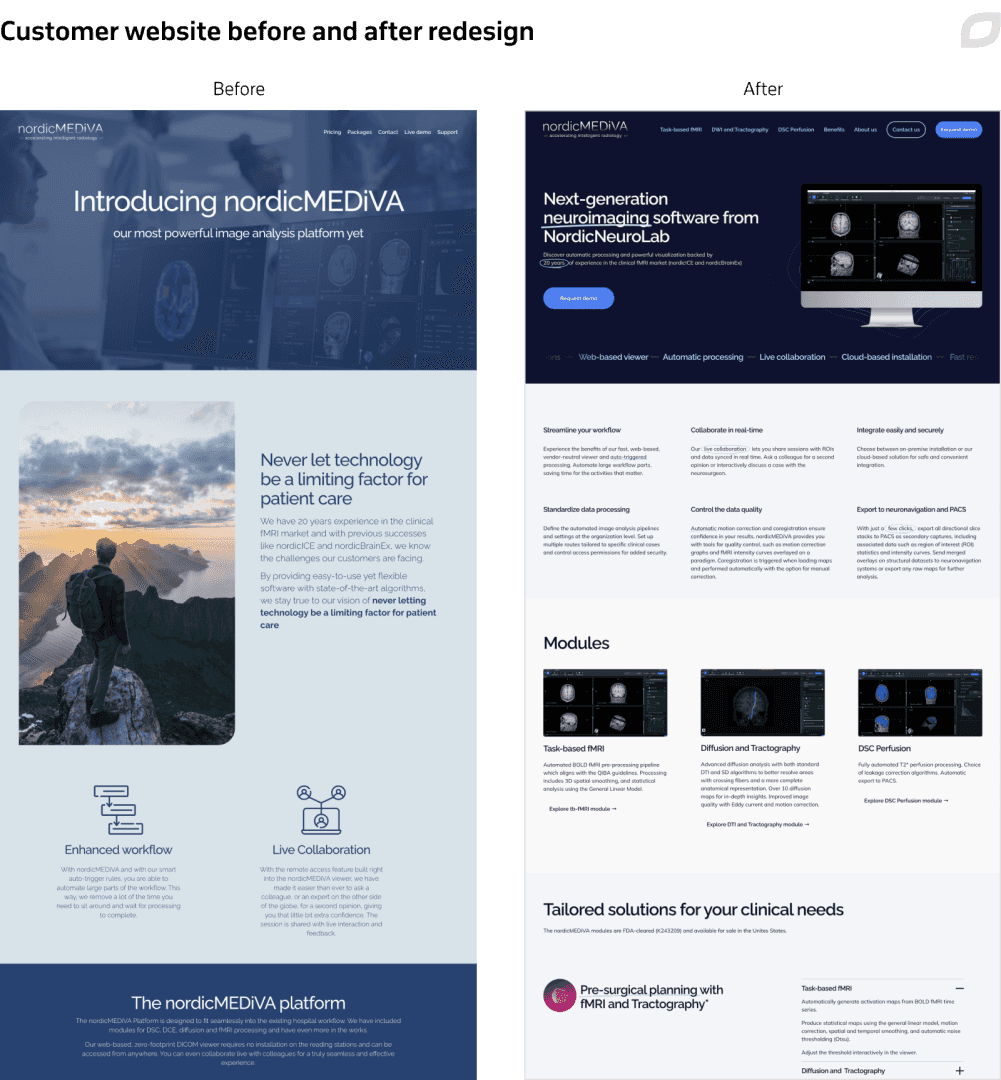
Additionally, Itransition completely redesigned the product’s website, updating both its design and content to attract clients. We also aligned the website’s and product’s designs.
Additionally, Itransition reworked the website's navigation, updated the solution modules’ descriptions, and fashioned a number of new sections. To keep end clients informed and build lasting relationships that drive retention and growth, Itransition also designed and continuously updated the newsletter section on the customer’s website.
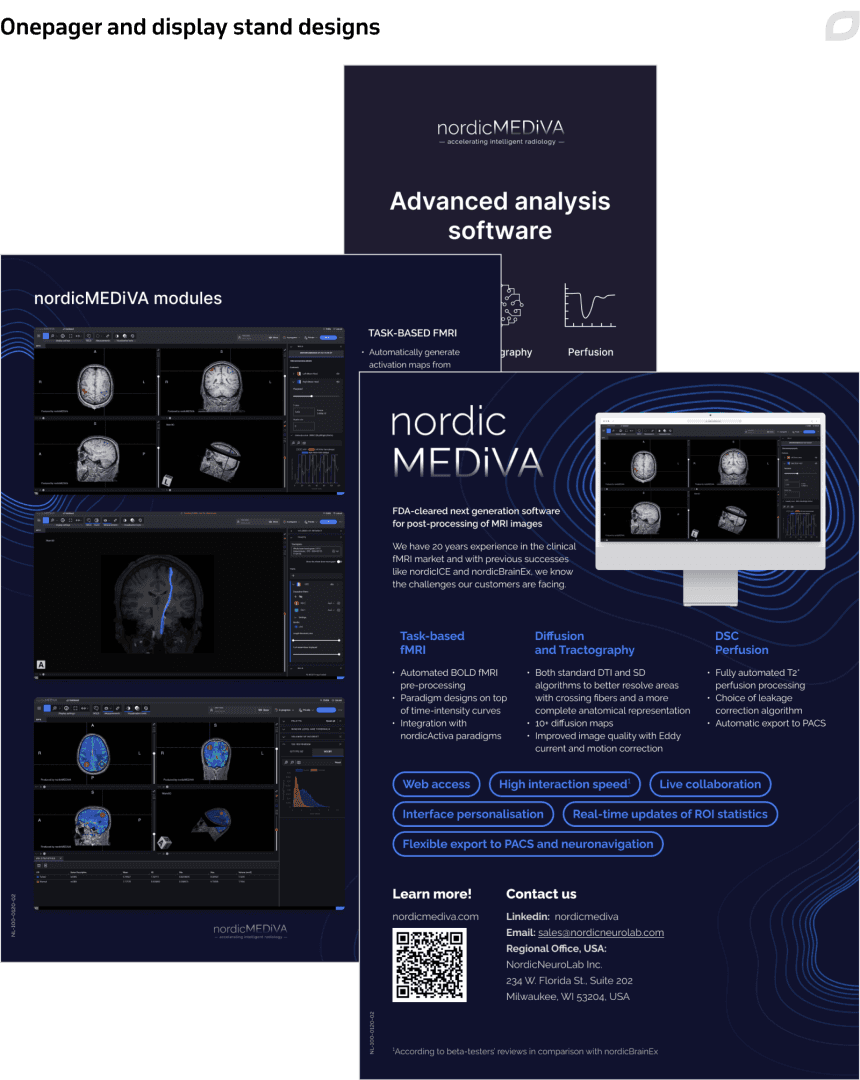
Furthermore, we created display stands and collaterals for conferences the customer participated in, supporting the company’s marketing efforts while entering the US market with the new product.
FDA clearance
With Itransition’s help, the customer completed two FDA submissions, both with their own set of different clinical and user experience challenges. However, the feedback for both submissions did not include any usability-related issues.
In line with the FDA requirements and recommendations, before the submission the customer and Itransition conducted formative and summative usability testing of the solution performed with the help of domain professionals (neurosurgeons and radiologists). Formative testing identifies what needs to be adjusted before summative testing. Summative usability testing is final, ideally conducted one time, and is required by FDA as opposed to the formative one. We also had to provide plans and reports for summative usability testing as part of the submission documentation. Our expert was responsible for planning, conducting usability testing, and reporting on it together with the product owner.
As part of getting the FDA clearance, we also conducted workshops with the risk management team to identify, describe, and categorize hazard-related use scenarios. We used data on potential risks that were documented during the exploratory interviews with radiologists. During the workshop, the project manager summarized potential risks, the customer’s company CEO, product owner, and other subject-matter experts were primarily responsible for risk detection and description, and Itransition participated as an invited expert. Summative usability testing included identifying potential hidden design risks, but none were found.
Results
Our collaboration resulted in a successful FDA clearance of the customer’s software product and meeting the agency’s stringent usability requirements. This milestone validated the product’s compliance with regulatory standards and confirmed its readiness for the US market.
With FDA clearance in hand, the customer successfully launched their product in the United States, accessing that market and setting the stage for their further business growth. Users highly appreciated the solution’s remote access, interaction speed, rendering quality, ease of use, and other capabilities. In Q1 of the year the customer received FDA clearance, their orders rose over 200% year-on-year.
Additionally, following an update to the customer’s website, page views surged by 90% and the number of leads generated through the site increased by a significant factor, further fueling the product's success in the market.

Services
Healthcare software development solutions & services
Entrust your medical software development to a company with 25+ years of experience in healthcare IT. Compliance and security guaranteed.

Case study
Unified care management platform
Learn how we delivered a healthcare platform that helps the client and their end-customers unify various care management workflows and enhance service quality.

Case study
Healthcare automation platform with EHR capabilities
Learn how Itransition developed a customizable automation platform to help healthcare professionals streamline manual tasks, reduce costs, and save time.

Case study
Atlassian suite optimization for a pharma solutions provider
Learn how Itransition helped a pharma provider fine-tune their Atlassian setup and migrate to Atlassian Cloud Enterprise, ensuring license costs optimization.

Case study
Medical IoT solution for emergency care
A multi-tenant HIPAA- and FDA-compliant solution for patient treatment during the Code Blue event and resuscitation cart inventory management. Read more.

Case study
Telepsychiatry platform for remote patient assessment
Learn how Itransition delivered a telepsychiatry solution for medical case processing, remote psychiatric assessment, and treatment recommendation delivery.

Case study
A BPM solution for medical staffing services
Discover how Itransition delivered a custom Odoo-based CRM/ERP/HR suite for a US medical staffing company.